INSPIOLTO RESPIMAT

TRONARTO study design:
Change from baseline in FEV1 AUC0–3h and trough FEV1 (secondary endpoint) at week 4
In a 4-week, randomized, double-blind, placebo-controlled, multi-centre, parallel group study which included 213 patients with moderate-and-severe COPD who were stratified according to their peak inspiratory flow (PIF) at screening (PIF ≥60 L/min or PIF <60 L/min), patients were randomized (1:1) to tiotropium/olodaterol 5 μg/5 μg daily dose or matching placebo, and received 2 actuations once daily. Treatment with tiotropium/olodaterol using the RESPIMAT Soft Mist™ Inhaler resulted in:
statistically significant improvements in FEV1 AUC0–3h and trough FEV1 versus placebo
Treatment difference after 4 weeks, by PIF subgroup
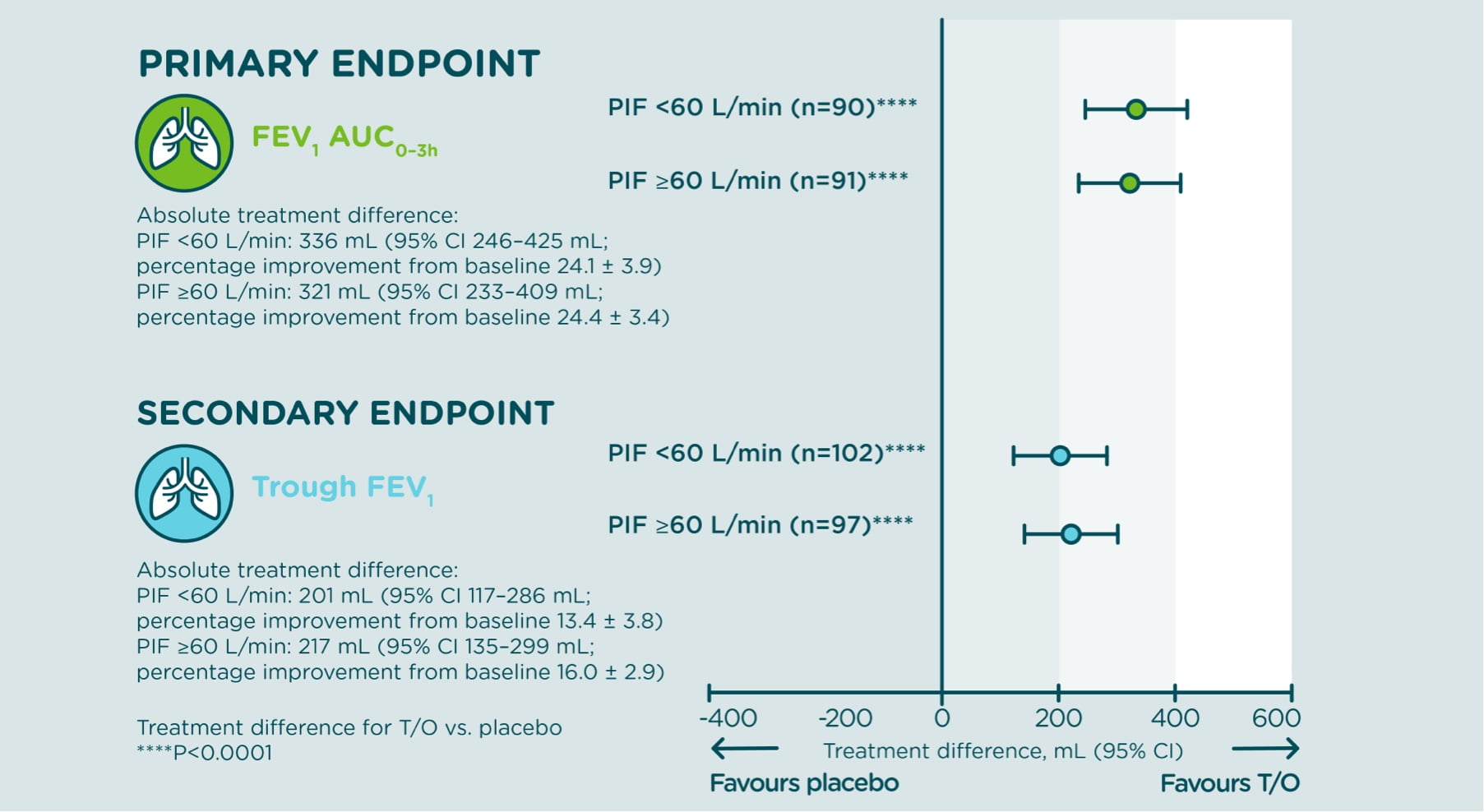
Adapted from Mahler D, et al. 2021.
Treatment difference after 4 weeks graph full text
AUC0–3h=area under the curve from 0–3 hours; CI=confidence interval; FEV1=forced expiratory volume in 1 second; PIF=peak inspiratory flow; T/O=tiotropium/olodaterol.
Quality of life data
In two studies: a greater proportion of INSPIOLTO RESPIMAT patients had an improvement in quality of life (QoL) vs. SPIRIVA RESPIMAT (secondary endpoint)*†
INSPIOLTO vs. SPIRIVA
Patients achieved a clinically meaningful improvement in the St. George’s Respiratory Questionnaire (SGRQ) total score at 24 weeks (n/N=465/955 [48.7%] for SPIRIVA RESPIMAT vs. n/N=563/979 [57.5%] for INSPIOLTO RESPIMAT; MCID: defined as a decrease of at least 4 units from baseline)
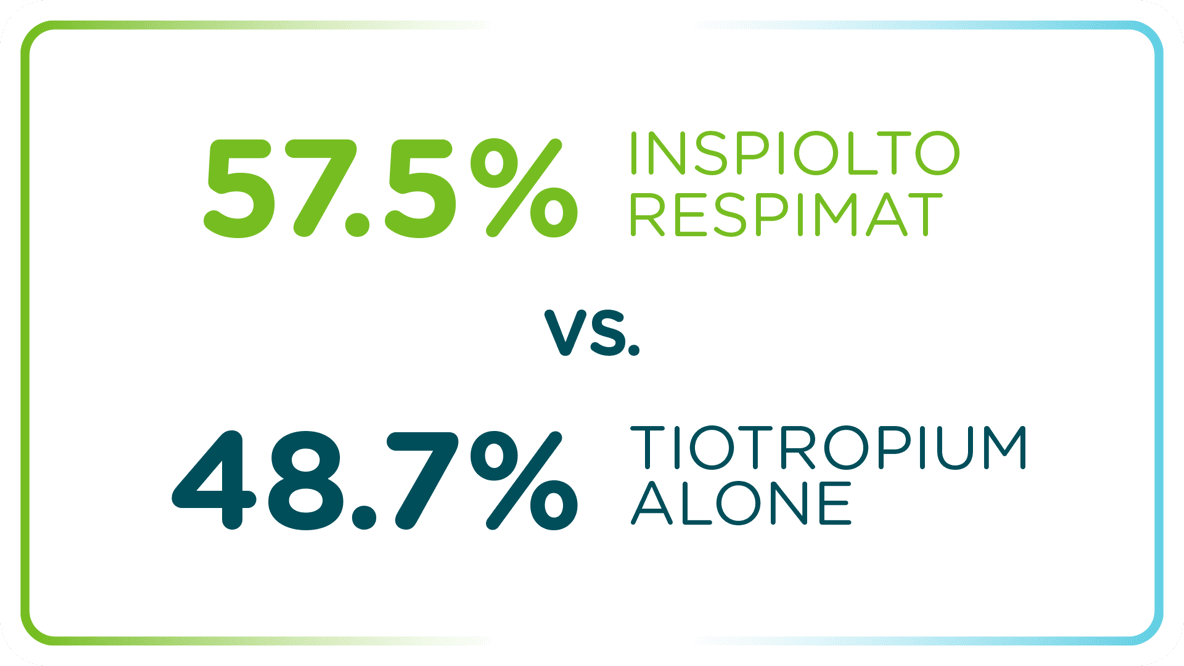
MCID=minimal clinical improvement difference.
-
*
Efficacy and safety of tiotropium+olodaterol fixed-dose combination (FDC) compared with the mono-components were evaluated in patients with moderate to very severe chronic obstructive pulmonary disease (COPD) in two replicate, randomized, double-blind, parallel-group, multicentre, phase III trials.
-
†
Scores range from 0–100, with higher scores indicating more limitations.
Breathlessness and exercise endurance data
In two studies: INSPIOLTO RESPIMAT showed improvements in breathlessness vs. SPIRIVA RESPIMAT (secondary endpoint)*

Measured by mean change in Transition Dyspnea Index (TDI) focal score on day 169 (absolute difference of 0.36 units; 95% CI: 0.09–0.62).
-
*
Efficacy and safety of tiotropium/olodaterol fixed-dose combination (FDC) compared with the mono-components was evaluated in patients with moderate to very severe chronic obstructive pulmonary disease (COPD) in two replicate, randomized, double-blind, parallel-group, multicentre, phase III trials.
Rescue medication use

In the MORACTO 1 study – INSPIOLTO RESPIMAT significantly improved inspiratory capacity
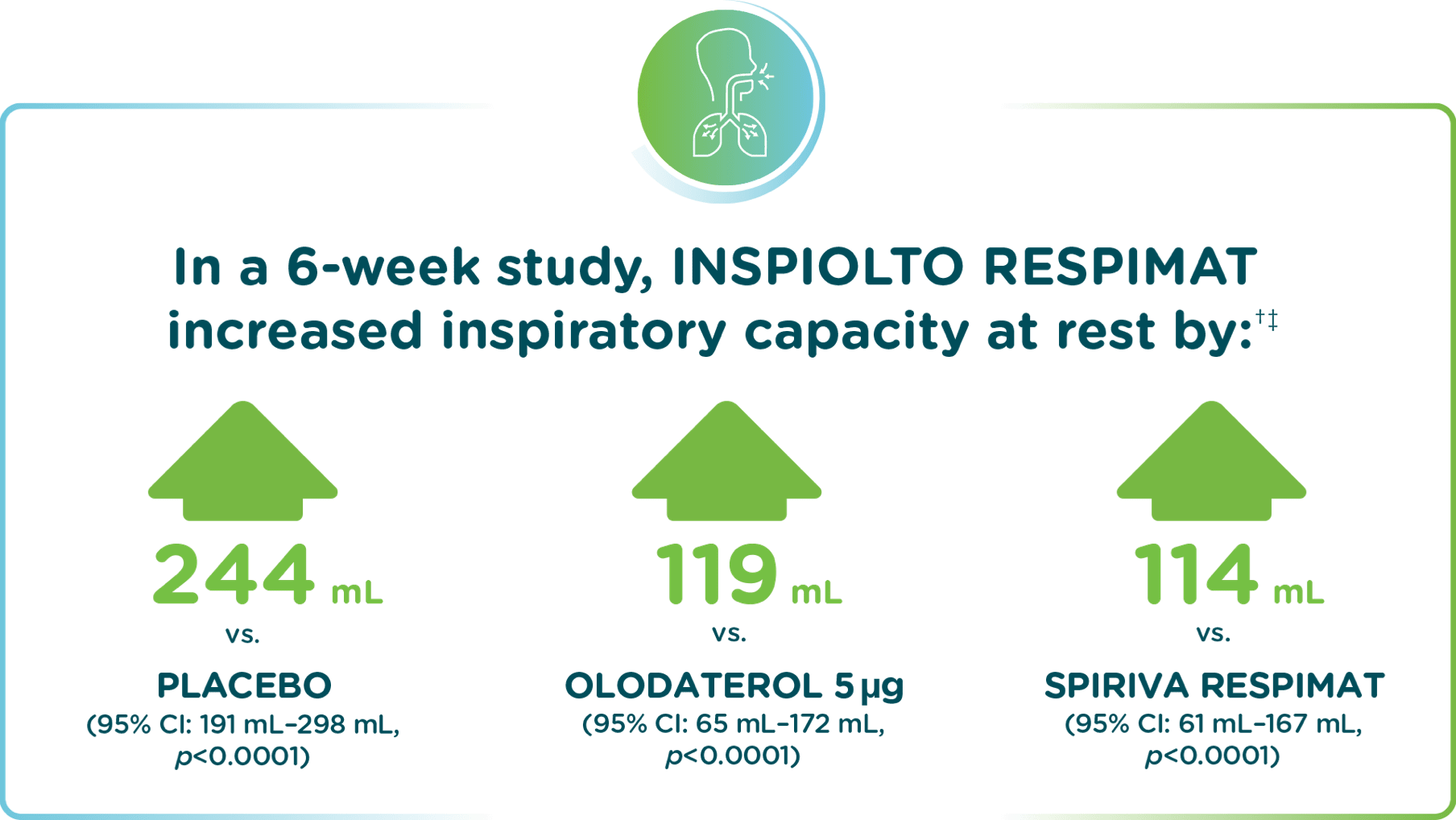
Adapted from INSPIOLTO RESPIMAT Product Monograph.
-
†
Two replicate, 6-week, randomized, double-blind, placebo-controlled, cross-over trials comparing INSPIOLTO RESPIMAT, SPIRIVA RESPIMAT, olodaterol 5 µg and placebo during constant work rate cycling (450 received INSPIOLTO RESPIMAT).
-
‡
Pre-treatment baseline of 2,530 mL for MORACTO 1.
In the MORACTO 2 study – INSPIOLTO RESPIMAT significantly improved inspiratory capacity
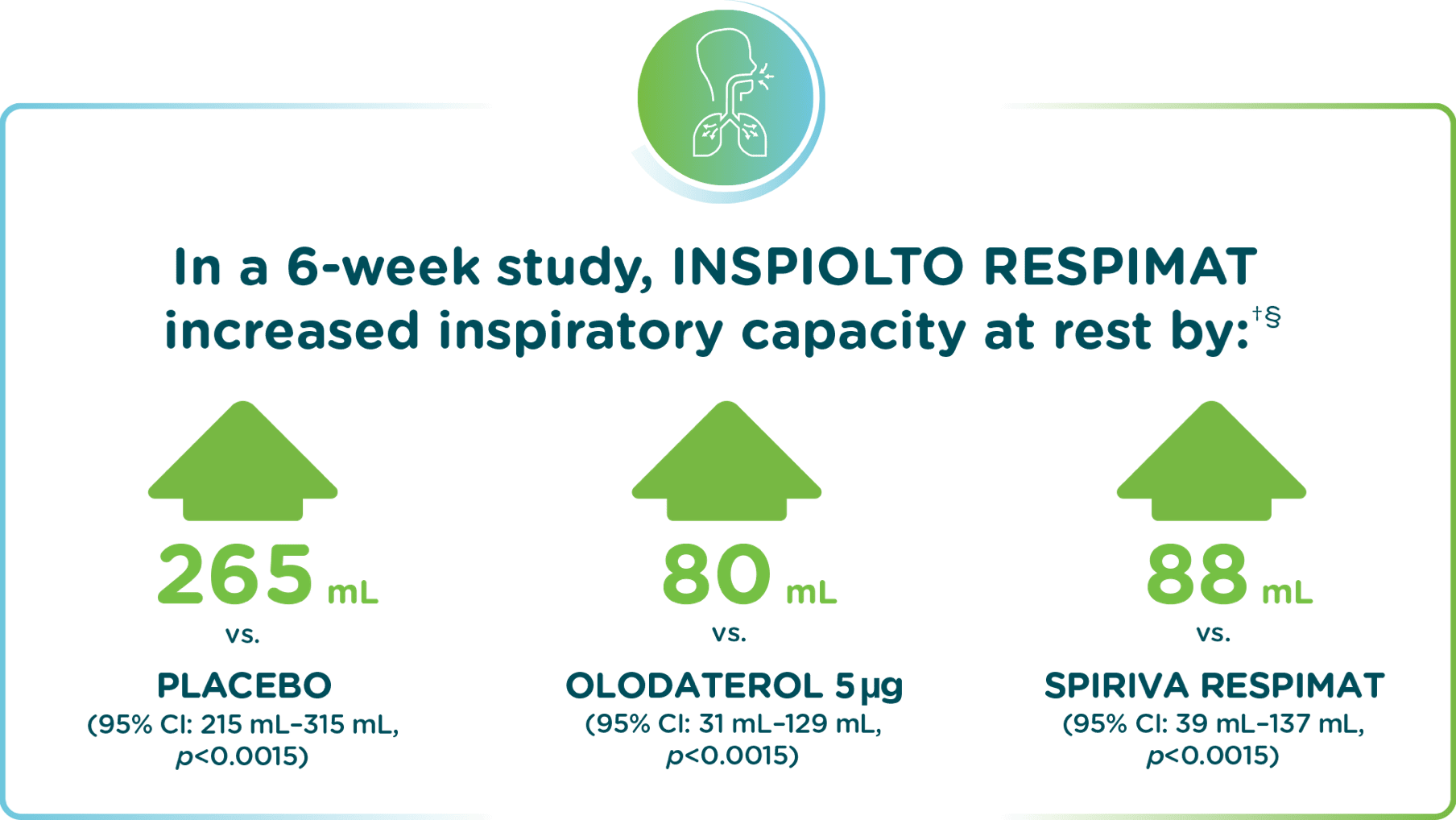
Adapted from INSPIOLTO RESPIMAT Product Monograph.
-
†
Two replicate, 6-week, randomized, double-blind, placebo-controlled, cross-over trials comparing INSPIOLTO RESPIMAT, SPIRIVA RESPIMAT, olodaterol 5 µg and placebo during constant work rate cycling (450 received INSPIOLTO RESPIMAT).
-
§
Pre-treatment baseline of 2,590 mL for MORACTO 2.
Lung function data
Clinical pharmacology: 24-hour bronchodilator profile*
INSPIOLTO RESPIMAT improved lung function (primary endpoint)†
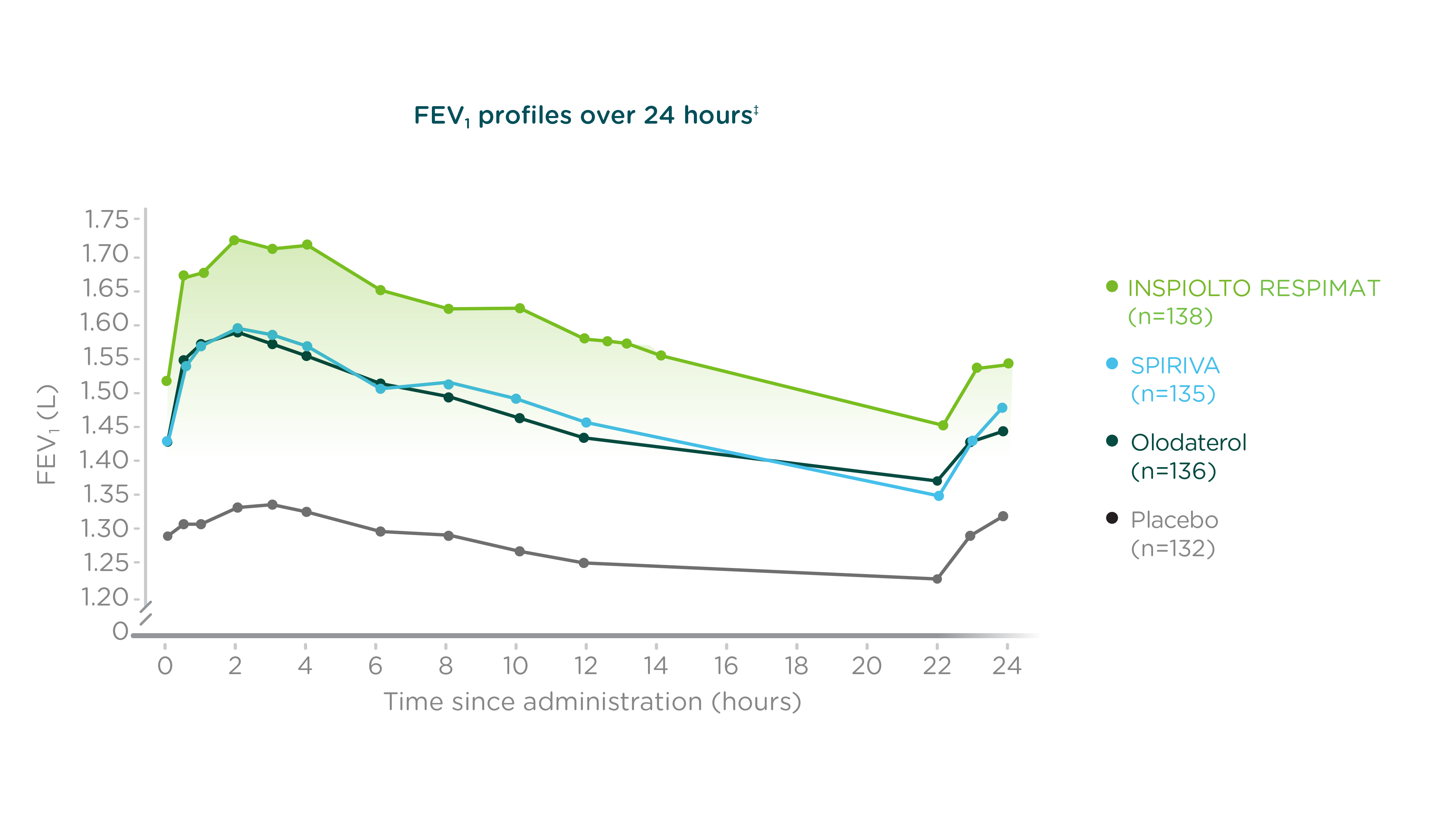
Adapted from INSPIOLTO RESPIMAT Product Monograph.
-
*
Comparative clinical significance has not been established.
-
†
Double-blind, placebo-controlled, multicentre, cross-over study in which patients were randomized to receive four of six treatment options for 6 weeks each: placebo, olodaterol 5 mcg, tiotropium 5 mcg, tiotropium + olodaterol FDC 5/5 mcg, all delivered via the RESPIMAT inhaler. Two of the six treatment arms were off-label; the results of these treatment arms have not been presented.
-
‡
24-hour FEV1 profiles after 6 weeks of treatment in the VIVACITO study. p<0.0001 for all comparisons of INSPIOLTO RESPIMAT vs. monotherapies and placebo.
INSPIOLTO RESPIMAT demonstrated significantly greater
FEV1 AUC0–24 vs. salmeterol/fluticasone at 6 weeks (secondary endpoint)§
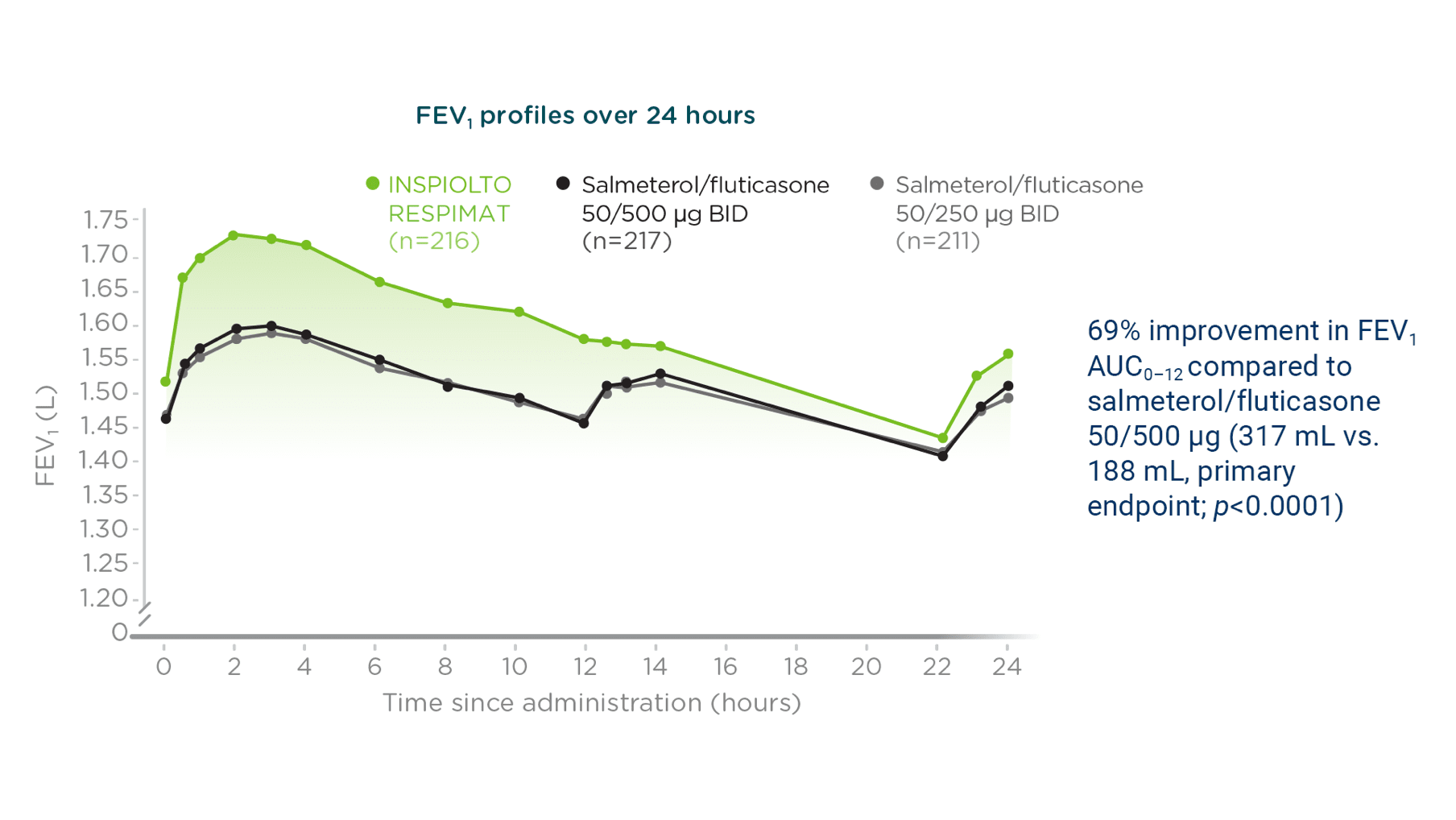
Adapted from Beeh K-M, et al. 2016.
-
§
A Phase IIIb, multicentre, multinational, randomized, double-blind, double-dummy, active-controlled, four-treatment, complete crossover trial. Patients were randomized to receive either INSPIOLTO RESPIMAT or salmeterol/fluticasone (50/500 μg or 50/250 μg) for 6 weeks.
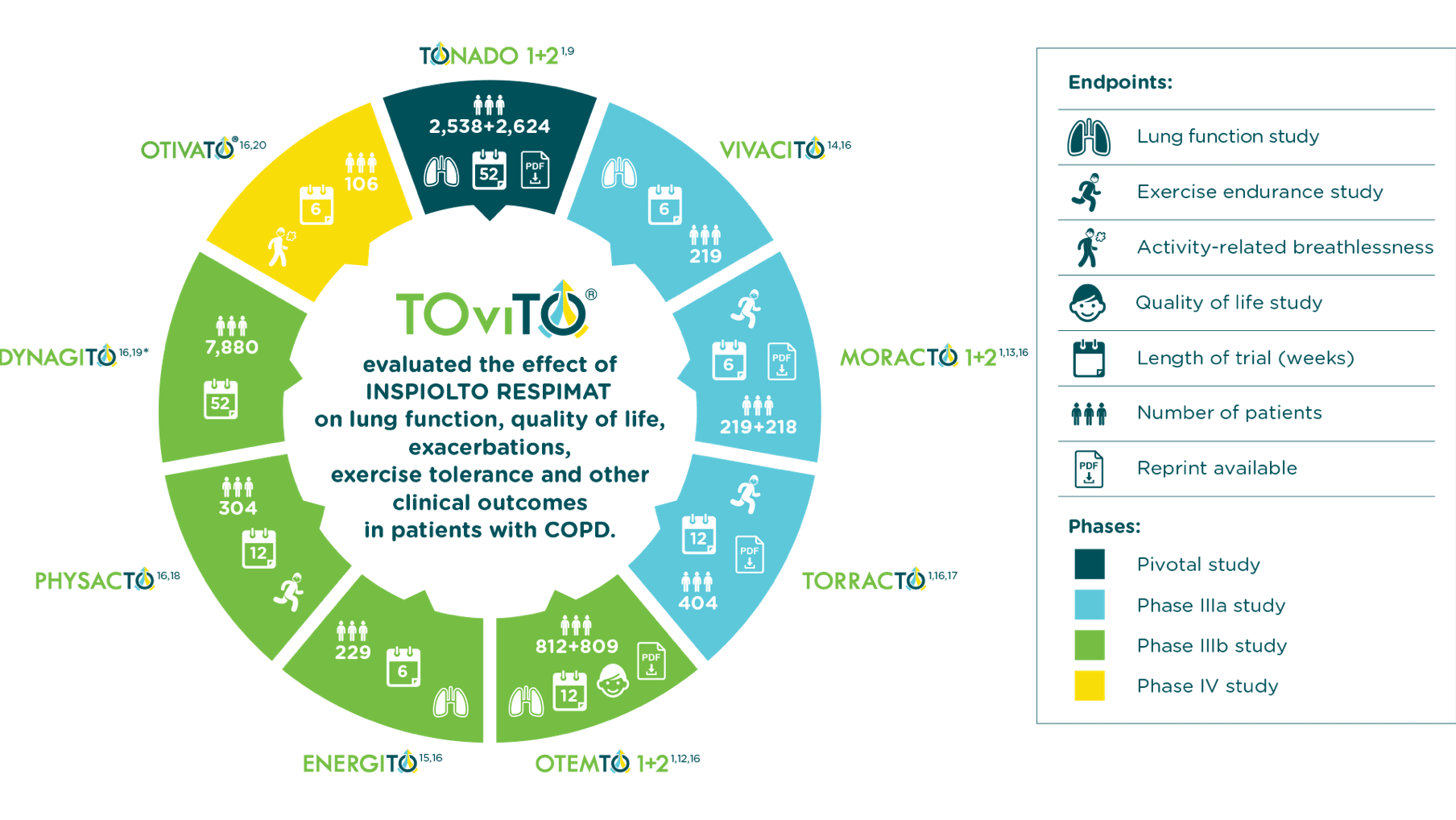
Clinical trials summary
With the TOviTO program, Boehringer Ingelheim studied over 15,000 patients worldwide in Phase III clinical trials
-
*
The DYNAGITO study did not meet the targeted 0.01 significance level (primary endpoint).