INSPIOLTO RESPIMAT
INSPIOLTO RESPIMAT (tiotropium bromide monohydrate and olodaterol hydrochloride) is a combination of a long-acting muscarinic antagonist (LAMA) and a long-acting beta2-adrenergic agonist (LABA) indicated for the long-term, once-daily maintenance bronchodilator treatment of airflow obstruction in patients with Chronic Obstructive Pulmonary Disease (COPD), including chronic bronchitis and emphysema.
It is not indicated for the relief of acute deterioration in COPD or asthma use.
Click here
for additional safety information and for a link to the Product Monograph discussing:
-
Contraindications in patients with hypersensitivity to tiotropium bromide monohydrate or olodaterol hydrochloride or to any of the excipients and patients with a history of hypersensitivity to atropine or its derivatives, e.g., ipratropium.
-
Most serious warnings and precautions regarding asthma-related death: Long-acting beta2-adrenergic agonists (LABA) increase the risk of asthma-related death.
-
Other relevant warnings and precautions regarding maximum daily use, asthma, treatment of acute episodes of bronchospasm, patients with acutely deteriorating COPD, use with other LABAs or LAMAs, narrow-angle glaucoma or urinary retention, avoiding getting mist into eyes, cardiovascular disorders, patients predisposed to low levels of serum potassium, patients with known history of QTc prolongation, patients with convulsive disorders or thyrotoxicosis and patients who are unusually responsive to sympathomimetic amines, patients with preexisting diabetes mellitus or ketoacidosis, dizziness or blurred vision, cardiovascular effects such as increase in pulse rate, systolic or diastolic blood pressure or cardiac arrhythmias, wheezing and breathing difficulties (bronchospasm) due to benzalkonium chloride, paradoxical bronchospasm, use in pregnant and nursing women, hepatic insufficiency and renal insufficiency.
-
Conditions of clinical use, adverse reactions, drug interactions and dosing instructions.
The Product Monograph is also available by calling us at 1-800-263-5103 ext. 84633.
SPIRIVA RESPIMAT
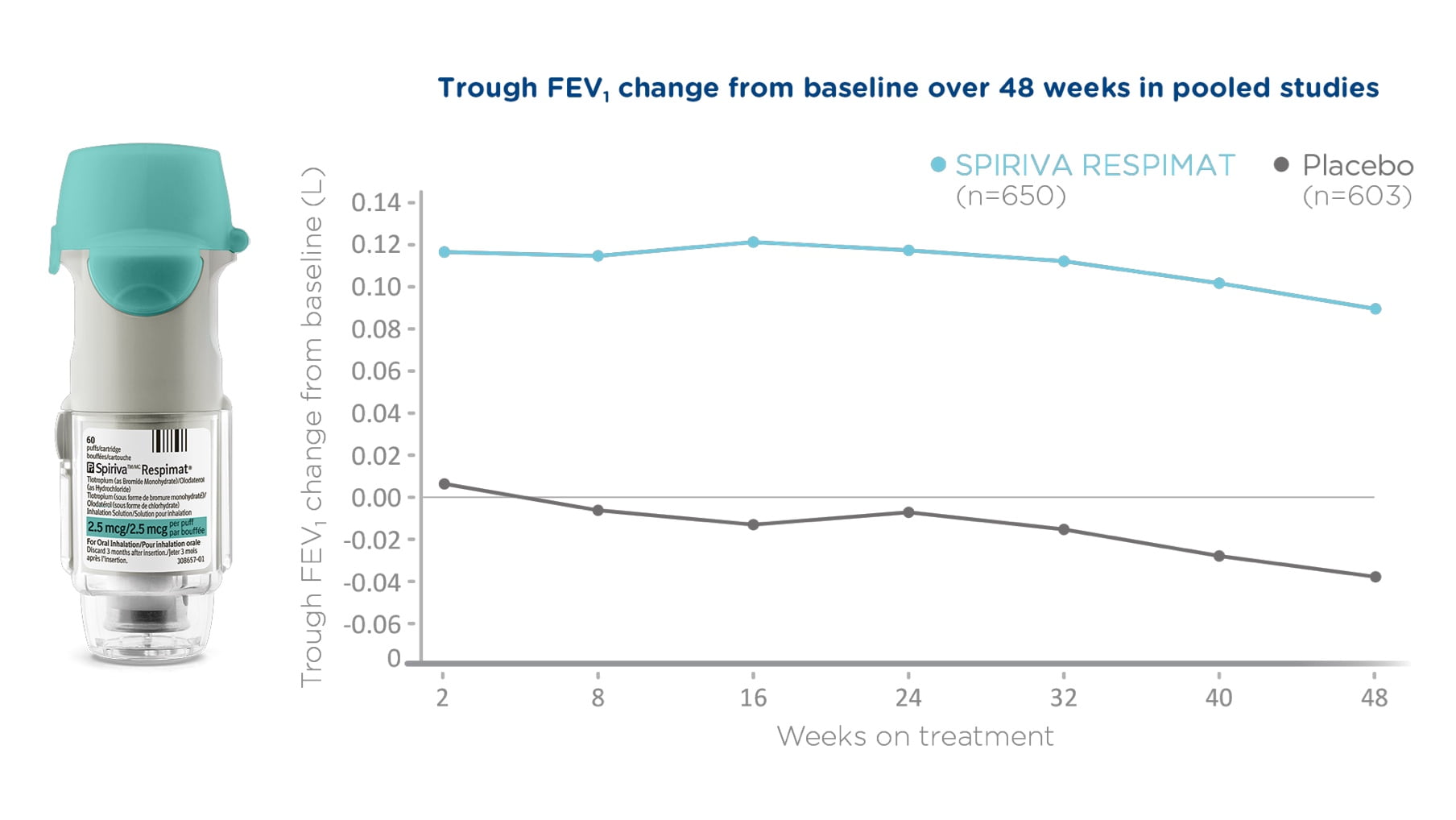
SPIRIVA RESPIMAT (tiotropium bromide monohydrate) is indicated as a long-term, once-daily maintenance bronchodilator treatment of airflow obstruction in patients with Chronic Obstructive Pulmonary Disease (COPD), including chronic bronchitis and/or emphysema, and for the reduction of exacerbations.
SPIRIVA RESPIMAT is indicated as add-on maintenance bronchodilator treatment in adult patients with asthma who remain symptomatic on a combination of inhaled corticosteroid (equivalent to, but not limited to ≥500 mcg fluticasone/day or ≥800 mcg budesonide/day) and a long acting β2-agonist and who experienced one or more severe exacerbations in the previous year.
SPIRIVA RESPIMAT is not indicated as rescue medication for the relief of acute bronchospasm in COPD or asthma.
Click here
for additional safety information and for a link to the Product Monograph discussing:
-
Contraindications in patients with a history of hypersensitivity to tiotropium bromide, atropine or its derivatives (e.g., ipratropium).
-
Other relevant warnings and precautions regarding initial treatment of acute episodes of bronchospasm or for the relief of acute symptoms, patients with acutely deteriorating COPD, which may be a life-threatening condition, first-line treatment or monotherapy for asthma, immediate hypersensitivity reactions, maximum use of two inhalations once daily, narrow-angle glaucoma, urinary retention (prostatic hyperplasia or bladder neck obstruction), avoiding getting mist into eyes, use with other LAMAs, dizziness or blurred vision, cardiovascular effects, such as cardiac arrhythmias, patients with moderate to severe renal impairment, wheezing and breathing difficulties due to benzalkonium chloride, induced bronchospasm, and use in pregnant and nursing women.
-
Conditions of clinical use, adverse reactions, drug interactions and dosing instructions.
The Product Monograph is also available by calling us at 1-800-263-5103 ext. 84633
COMBIVENT RESPIMAT
COMBIVENT RESPIMAT (ipratropium bromide and salbutamol sulfate) inhalation solution is indicated for treatment of bronchospasm associated with chronic obstructive pulmonary disease (COPD).
Click here
for additional safety information and for a link to the Product Monograph discussing:
-
Contraindications in patients with a history of hypersensitivity to ipratropium bromide or salbutamol sulfate or to atropine and its derivatives or in patients with cardiac tachyarrhythmias, and hypertrophic obstructive cardiomyopathy.
-
Other warnings and precautions for uncontrolled diabetes mellitus, recent myocardial infarction, severe organic heart or vascular disorders, hyperthyroidism, pheochromocytoma, risk of narrow-angle glaucoma, prostatic hypertrophy, urinary retention, coronary insufficiency, arrhythmias and hypertension, convulsive disorders and in patients who are unusually responsive to sympathomimetic amines, concomitant use with other sympathomimetic agents and muscarinic antagonists, hypertrophic subvalvular aortic stenosis, hyperglycemia and hypokalemia, gastrointestinal motility disturbances in patients with cystic fibrosis, hypersensitivity reactions, paradoxical bronchospasm, dyspnea, and use in pediatric and geriatric patients and pregnant or nursing women.
-
Conditions of clinical use, adverse reactions, drug interactions and dosing instructions.
The Product Monograph is also available by calling us at 1-800-263-5103 ext. 84633.
SPIRIVA HANDIHALER
SPIRIVA (tiotropium bromide monohydrate) is indicated as a long-term once-daily maintenance bronchodilator treatment of airflow obstruction in patients with Chronic Obstructive Pulmonary Disease (COPD), including chronic bronchitis and/or emphysema.
Click here
for additional safety information and for a link to the Product Monograph discussing:
-
Contraindications in patients with a history of hypersensitivity to tiotropium bromide, atropine or its derivatives (e.g., ipratropium), or to any component of this product.
-
Other warnings and precautions for galactose intolerance, total lactose deficiency, glucose-galactose malabsorption, acute use, COPD deterioration, excessive use, anticholinergic effect, cardiovascular effect, driving and operating machinery, hypersensitivity reaction, worsening of narrow-angle glaucoma, use in moderate to severe renal impairment, worsening of urinary retention, paradoxical bronchospasm, and use in pediatric and geriatric patients and pregnant or nursing women.
-
Conditions of clinical use, adverse reactions, drug interactions and dosing instructions.
The Product Monograph is also available by calling us at 1-800-263-5103 ext. 84633.